How To Go From Newman Projection To Line Drawing
Determining R and South Configurations of Newman Projections
How do you determineR andS configurations on Newman projections? The key is to be able to chop-chop convert Newman projections into line diagrams, so use the familiar CIP rules to decide R/Due south on the line diagrams.
And then how practice yous catechumen Newman projections to line diagrams? That's what we're going to comprehend in this article.
Tabular array of Contents
- Determining (R) and (Due south) On Newman Projections: A Good Thing To Know For A Stereochemistry Exam
- Near People Are Actually OK At Visualizing Familiar Things In three-D. The Problem For Beginners In Organic Chemistry Is That Molecules Are Not Familiar
- Cats Are Familiar. So Let's Draw The "Newman Projection" Of A Cat
- The Newman Project: Eclipsed and Staggered Conformations
- Eclipsed And Staggered Conformations Are Interconverted By Rotation Of threescore° Along The Central C-C Bail
- How To Convert A Newman Projection To A Line Diagram
- A Cheat Canvas For Going From A Newman To A Line Diagram: There Are Only four Templates To Consider
- Determining R and S Configuration On Newman Projections: Example #one
- Determining R and S Configuration On Newman Projections: Example #2
- Determining R and Due south On A Newman With An Eclipsed Conformation: Example #three.
- Conclusion: Determining R and S Configurations In Newman Projections
1. Determining (R) and (S) On Newman Projections
In two recent posts we discussed how to utilize the Cahn-Ingold-Prelog (CIP) rules to assign (R/South) to configurations of chiral carbons in a diverseness of situations, both simple and more complex.
And so far, all the questions have asked you to assign (R/South) on molecules drawn every bit bail-line diagrams, such as the molecule shown lesser left.
This is fine. Only every once in awhile – like on an exam, for instance, hint hint – you lot might detect yourself thrown for a loop. For instance, how do you determine R/S when the molecule is fatigued as a Newman? (bottom right)
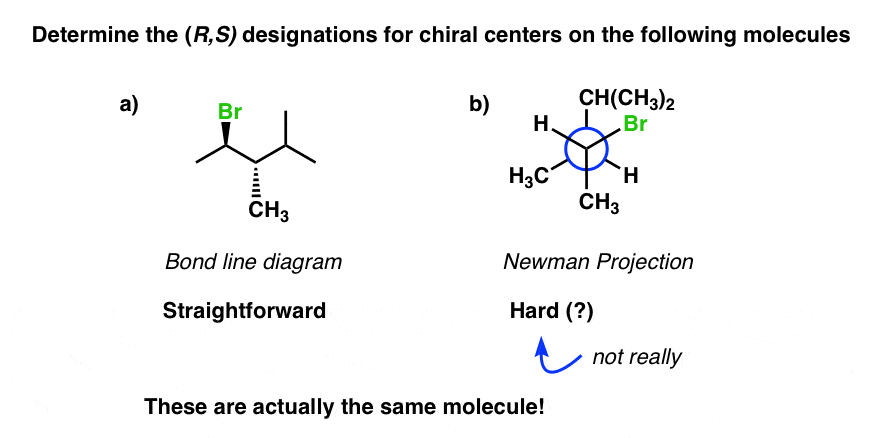
The fob is to convert the Newman project to the bond-line diagram and then assign R/Due south.
This post explains how to do that.
This post was co-authored with Matt Pierce of Organic Chemistry Solutions. Inquire Matt about scheduling an online tutoring session hither.
2. Most People Are Really Pretty Expert At Visualizing Familiar Things In 3-D. The Problem For People Starting Organic Chemistry Is That Molecules Are Not Familiar
Ane common thing I hear from students about why organic chemistry is difficult is that they say they have "a hard time visualizing things in 3D".
I actually don't think this is truthful.
I think most people are fine visualizing things in 3D.
The problem is that visualizing molecules is unfamiliar.
Given this hypothesis, let's take something that is familiar and do some visualization exercises.
Here'south a picture of a hungry Jerusalem street cat.

Could y'all visualize what it would look like from the side?
Almost certainly, because you are very familiar with how cats look from virtually angles.
If yous had to make a cartoon (stick figures are fine) it would probably look something similar this:
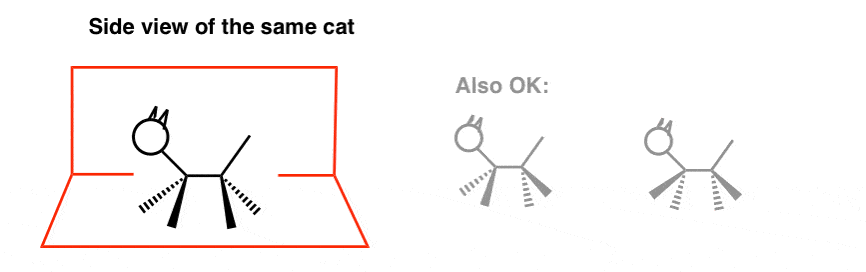
Note nosotros took some liberties. The legs facing us are fatigued as wedges and the ones pointing away are dashes.
[Here, I drew the two wedges on the "inside" relative to the dashes, but cartoon them on the exterior (or even alternating) is OK, since information technology amounts to the same thing]
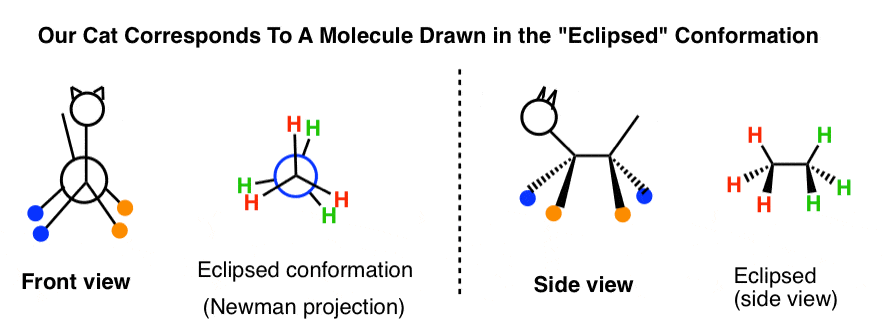
3. The "Newman Project" Of A True cat
Now let'due south do the aforementioned kind of exercise, but in reverse.
Let'southward take that the stick figure nosotros only drew and try to picture what it would await like from the front (i.e. look from the left) and from the back (await from the right).
For reasons that volition soon become apparent, we'll add a bit of detail: let'south requite the true cat some colored "socks" (orangish and blue).
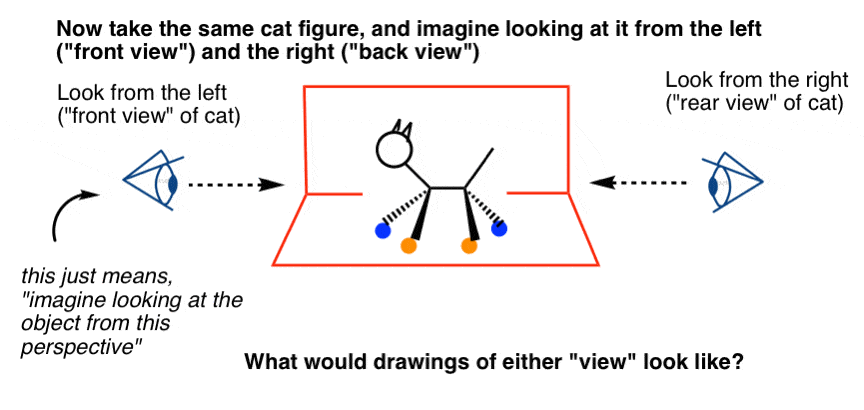
[Yous might ask: what's that weird looking symbol? Information technology'due south the Side-Eye of the Illuminanti, the symbol of the underground underground society of chemists that rules the world just a symbol that says, "imagine looking at this thing from this direction"]
Because you lot likely have avery good iii-D mental model of a true cat, y'all shouldn't accept constitute practice this as well difficult.
Hopefully you got something like this, below. For simplicity, I omitted drawing in the eyes [2 in the front view, 1 in the back view (heh)]
The circle represents the true cat'south body, since the front and back hips block each other.
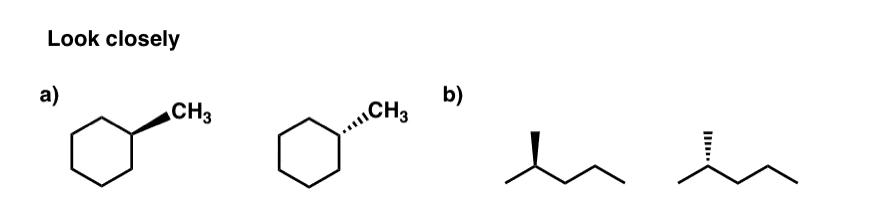
Maybe you noticed this helpful correspondence:
- When we looked at the cat from theleft (i.e. front view) the groups on wedges (orange) concluded up on theright side.
- When we looked at the cat from theright(i.e. back view) the groups onwedges(orangish) ended up on theleft side
4. The Newman Projection: Eclipsed and Staggered Conformations
Of course this has all just been a roundabout way of reviewing the Newman project, too as an do in trying to assistance you realize that yous are meliorate at visualizing molecules in 3-D than you previously may have thought.
It helps that cats map on to molecules pretty well!
Recall that Newman projections are a user-friendly style of showing conformations in molecules. For instance, the cat nosotros only drew was in the "eclipsed" conformation, where the head and tail both line up with each other like the hour and minute hands on a clock striking midnight. The front and dorsum legs line upwards as well.
The other pregnant conformation of note is the "staggered" conformation, where the front end iii groups are get-go past 60 degrees with respect to the dorsum three groups.
[Despite several attempts, I was unable to obtain a expert photo of a Jerusalem street true cat in a staggered conformation. They really don't like beingness twisted. So we'll have to piece of work with models.]
5. Eclipsed And Staggered Conformations Are Interconverted By Rotation Of 60° Along The Central C-C Bail
In the example beneath, we'll rotate the back carbon 60 degrees clockwise (CW) with respect to the front carbon, along the central carbon-carbon bond. After this is washed, notation how the light-green hydrogens accept moved from 12:00 to 2:00, 4:00 to half dozen:00, and 8:00 to 10:00 respectively.
When we await at this "staggered" molecule from the side, we obtain a bail-line diagram where the bonds in the aeroplane of the page take a zig-zag configuration (bottom correct).
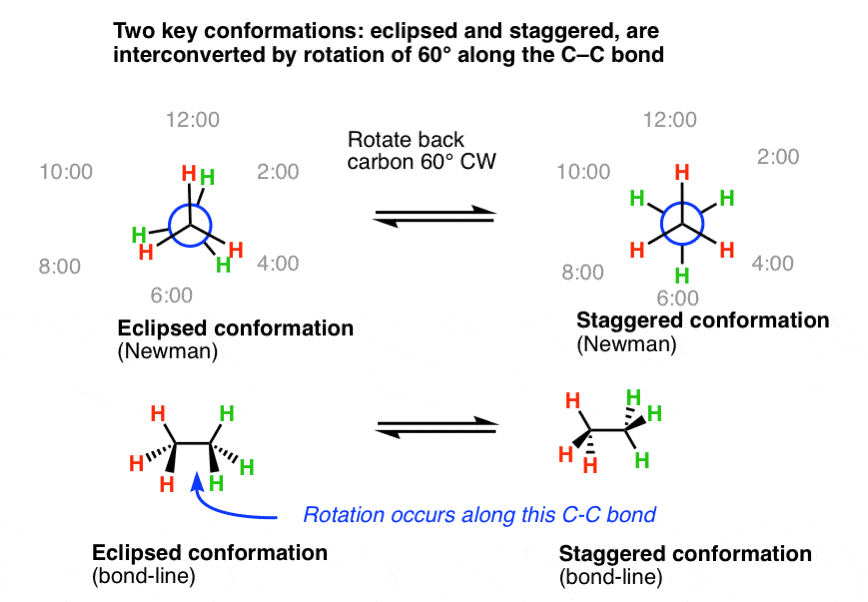
If we expect at this "staggered" bond-line diagram from the left, we obtain the "staggered" Newman, fatigued top right.
6. How To Catechumen A Newman Projection To A Line Diagram
So how do nosotros convert a Newman diagram to a bail-line diagram? This section will walk through all the steps.
The outset thing to recognize is that in bond-line diagrams there are only iv possible patterns that the bonds in the plane of the page will follow.
There are 2 possible "zig-zag" shapes, corresponding to the "staggered" conformation, and at that place are also two possible "C-shapes" corresponding to the "eclipsed" conformation. [Note that line diagrams are oftentimes tilted xxx° from these directions, but for simplicity nosotros're going to keep the central C-C bond strictly horizontal].
If we look from the left on each of those 4 line diagram patterns, we can see that each i generates a different Newman projection pattern.
In that location are 4 Newman projection patterns:
- front end downwards/support,
- front up/back down,
- front end up/back up,
- and front down/back down.
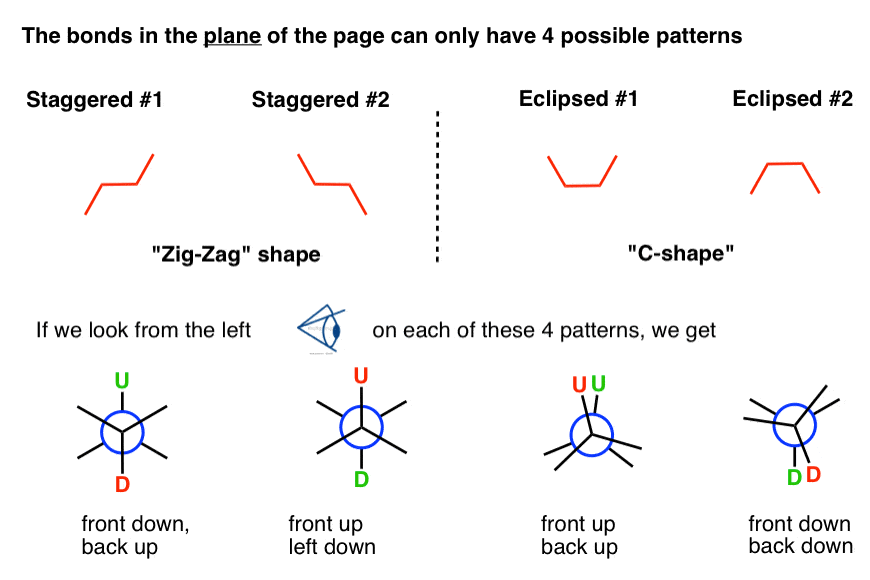
Now that we've seen how the patterns work in the frontward direction, let's at present employ these patterns in thecontrary direction.
7. A Cheat Canvas For Going From A Newman To A Line Diagram: There Are Merely 4 Templates To Consider
Using these templates, we can have any Newman project and work backwards to get the corresponding bond-line template, so draw in the dashes and wedges.
Here are the four Newman project patterns, converted into line diagrams. (On the right, y'all'll encounter what information technology looks similar when tilted 30°).
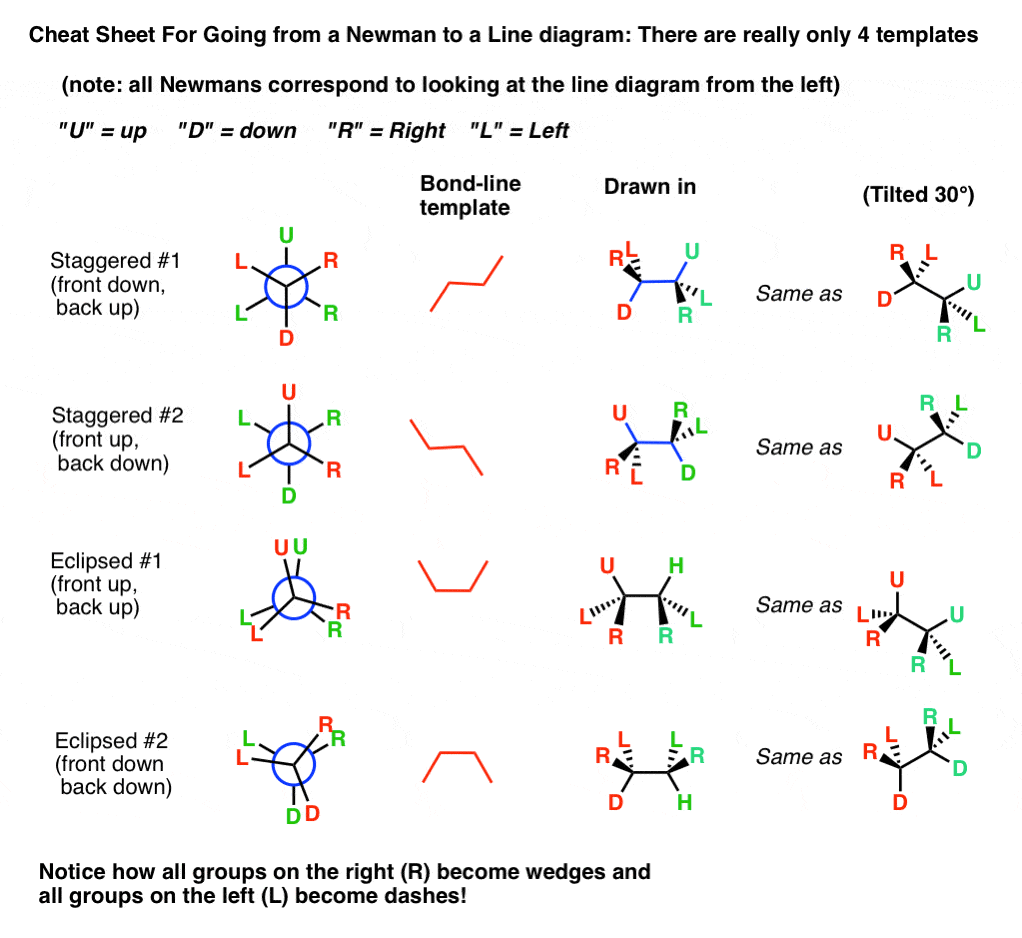
Ane important matter to note. Equally nosotros saw with the cat, when we look from the left side of the molecule:
- all groups on the right (R) become wedges, and
- all groups on the left (50) become dashes
If you follow through with the pattern of looking at the molecule from the left perspective, then all you lot demand to remember is to draw the wedges on the right side of the Newman diagram.
viii. Determining R and S Configuration On Newman Projections: Example #one
Let'south use this to a few specific examples.
First, let'southward assign R/Due south to a Newman fatigued in a staggered conformation with a single stereocenter.
This ane is drawn equally (front up, back down) so we will use Template #2 from above.
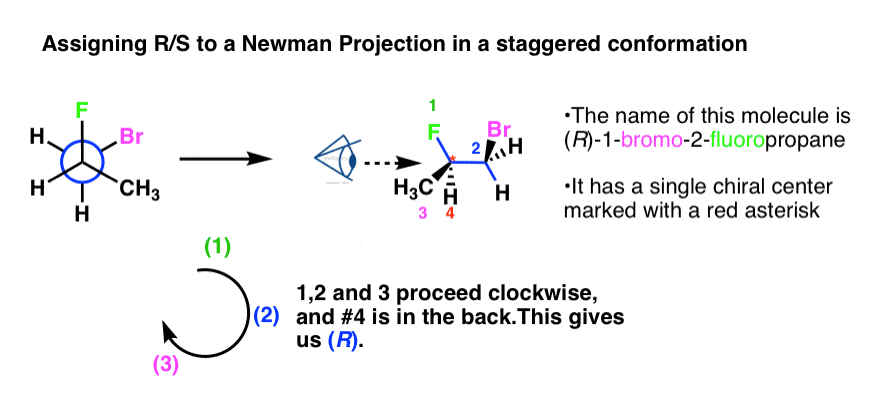
In this example we drew the (forepart upward, back downward) staggered template, and then filled in the bonds. Note that the groups on the right of the Newman (Br and CH3) became fastened to wedges in the line diagram.
Y'all should obtain (R) as the configuration.
9. Determining R and S Configuration On Newman Projections: Example #2
Next, let'due south go dorsum and do our original instance (2-bromo-3,4-dimethyl pentane).
It is also drawn in the staggered conformation (front down, dorsum up). So here nosotros volition utilizeTemplate #1.
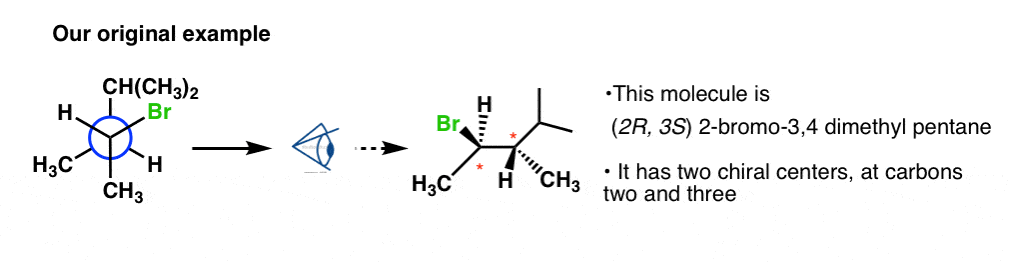
Using the aforementioned method, y'all should obtain (R) for the stereocenter containing Br and (S) for the stereocenter on carbon #iii. For details on how this was done, here's the prototype.
ten. Determining R and Due south On A Newman With An Eclipsed Conformation: Example #3.
What if the molecule is in an eclipsed conformation? Endeavour this one.
This follows the (front end downwardly, back down) pattern, so follow Template #4
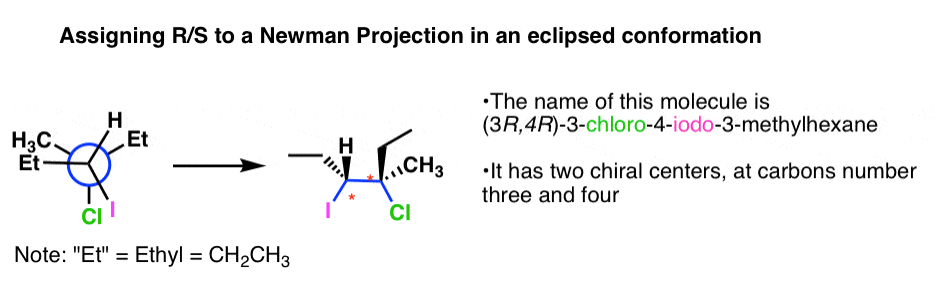
You should obtain (3R, 4R). To see details of how it was done, click hither.
11. Conclusion: Determining R and S Configurations In Newman Projections
If yous tin can visualize what a cat would await like from the front and from the side, and then yous should be able to convert a Newman projection to a line diagram. This is the showtime footstep in determining R/S on a Newman projection.
Knowing that there are only a few templates makes it easier.
Once you do information technology plenty times, you won't fifty-fifty need the templates, and yous might find that information technology'due south easier to simply do it in your head.
Comments or questions? Please ask!
In the next post, we'll expect at the Fischer project.
Cheers again to Matt for helping with this post. Hire Matt as your tutor!
Source: https://www.masterorganicchemistry.com/2017/02/01/assigning-rs-to-newman-projections-and-converting-newman-to-line-diagrams/
Posted by: lafranceshearompal.blogspot.com


0 Response to "How To Go From Newman Projection To Line Drawing"
Post a Comment